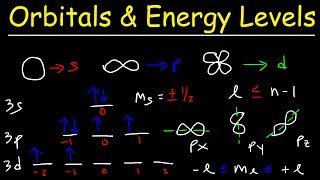
How Many Different Sublevels Are in the Second Energy Level
Each principal energy level above the first contains one s orbital and three p orbitals. How many total orbitals are in the second energy level.
How Many Different Sublevels Are In The Second Energy Level At Level
Maximum l values is n-11.
. 8 Electrons is the most the 1st energy level can hold. Absorbs a continuously variable amount of energy c. The Second Principle Energy Level can hold a maximum of how many Electrons.
The third principle energy level has three sub-levels 3s 3p. These orbitals will be distributed on two subshells the s-subshell and the p-subshell. Sub-levels all have different.
You can understand it by thinking about different things. Every energy level except the first level contains three P-Orbitals. It is written as 1s.
So for n 2. These sublevels hold different amounts of electrons. Q1Which sublevels are contained within the second energy level.
L 0 and 1 corresponding to s and p orbitals. Each subshell has a different energy level or sublevel. The first shell can hold one sublevel s.
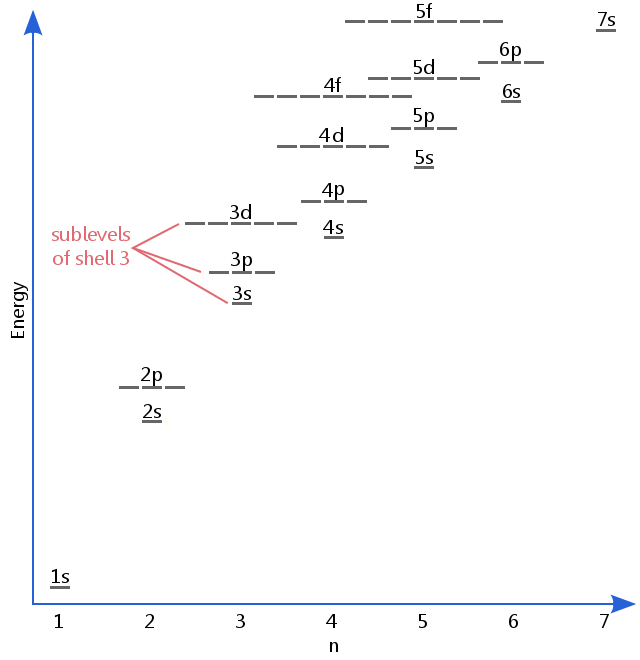
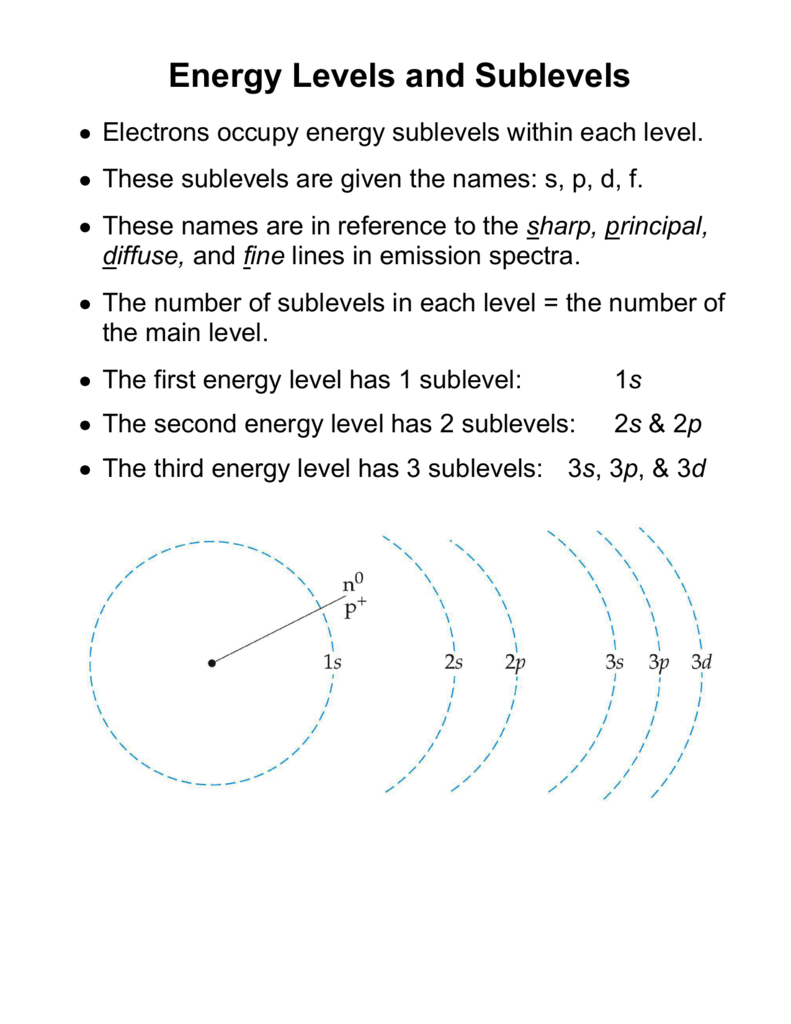
Up to 24 cash back An S-Orbital in the second energy level is a 2s orbital etc. Up to 24 cash back Energy Level Names of sublevels that exist in the energy level 1st energy level s 2nd energy level s and p 3rd energy level s p and d 4th energy level s p d and f Note that there is no such thing as a d sublevel inside of the 2nd energy level because there are only s and p sublevels inside of the 2nd energy level. There are 2 possible types of orbitals for energy level 2.
Three orbitals in the p-subshell. Answer Q3How many electrons does it take to fill the 10th energy level Answer Q4 How many orbitals are in each type of sublevel s p d 1. Expert Answer 100 12 ratings We have second energy level 2 That means principal quantum number is 2 or.
S only has one possible orientation. The energy levels are numbered that of the lowest energy being denoted by n1 and that of the next greatest being n2 and so on. Because the sub levels or so close to one another that they switch where the n value is lower so the electrons fills group d before s.
2nxn The main energy level number the shell number is known as the principal quantum number. For 3rd energy level n 3Maximum number of electrons in the 3rd energy level 2n2 2x 3 2 2 x 9 18. View the full answer Previous question Next question.
These two energy sublevels are 2s and 2p. They exist in groups of three. See the answer how many sublevels are contained in the second energy level n2 of a given atom.
Answer Q2How many orbitals are in the 9th energy level. Absorbs a quantum of energy d. Level 3 has 3.
1 how many energy sublevels are in the second. Up to 24 cash back For 2nd energy level n 2Maximum number of electrons in the 2nd energy level 2n22 x 22 2 x 4 8. A set of three p orbitals called the p sublevel can hold a maximum of six electrons.
Therefore the second level can contain a maximum of eight electrons - that is two in the s orbital and 6 in the three p orbitals. When an electron moves from a lower to a higher energy level the electron ____. Level 2 has 2 sublevels - s and p.
Lowest energy to highest. The second shell has two subshells and is written as 2s 2p. 4 rows Level one has one sublevel an s.
The further designations after f are in alphabetical order. The maximum number of electrons that can fit into the 2nd energy level is. This problem has been solved.
X y and z. These sublevels have the same shape. S sublevelorbitals p sublevelorbitals d sublevelorbitals f.
Therefore it can hold a maximum of two electrons. M magnetic quantum number which refers to the orientation of each orbital in space. One orbital in the s-subshell.
How many energy sublevels are in the second principal energy level. Notice that the sub levels for group d and s changed for titanium that is. There are shaped like a 3D figure of eight.
The second energy level will thus have a total of 4 orbitals. The energy distribution in an atom is divided into a number of. Each Sub-Level is comprised of.
Since each orbital can hold a maximum of 2 electrons. But first lets be super clear. For 4th energy level n 4Maximum number of electrons in the 4th energy level 2n2 2x 4 2 2x16 32.
Therefore when n1 the electron capacity is2 and when n2 the electron capacity is 8 and when n3 the capacity is 18 ie. The second principle energy level has two sub-levels 2s and 2p. Electrons fill sub-levels in the order of.
Each Principle Energy Level is comprised of different Sublevels which are s p d f The s - Sublevel can hold a maximum of 2 Electrons The p - Sublevel can hold a maximum of 6 Electrons The d - Sublevel can hold a maximum of 10 Electrons The f - Sublevel can hold a maximum of 14 Electrons Sub-levels all have different Energies. These sublevels are the same distance from the nucleus. There are two energy sublevels in the second principal energy level.
You can thus say that the second energy level will contain. Always doubles its energy b. Related Question Answers Kardama Erfini Professional.
Each P-Orbital in the same energy level has the same energy but different orientations. Critical Thinking Questions 1. Carbon sub-levels are.
L 0 and all integers less than n-1. Each shell or principle energy level has a defined number of subshells it contains. The first principal energy level contains only an s sublevel.
1s2 2s2 2p6 3s2 3p6 3d2 4s2.
Orbitals Atomic Energy Levels Sublevels Explained Basic Introduction To Quantum Numbers Youtube
How Many Sublevels Are In The Second Energy Level At Level

Electron Configurations Ppt Download
Definition Of Sublevel Chemistry Dictionary

How Many Sublevels Are In The Second Energy Level At Level

How Many Electrons Are In The Second Energy Level At Level

How Many Different Sublevels Are In The Second Energy Level At Level

Sf2 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist
How Many Sublevels Are In The Second Energy Level At Level

How Many Sublevels Are In The Second Energy Level At Level

How Many Sublevels Are In The Second Energy Level At Level

Comments
Post a Comment